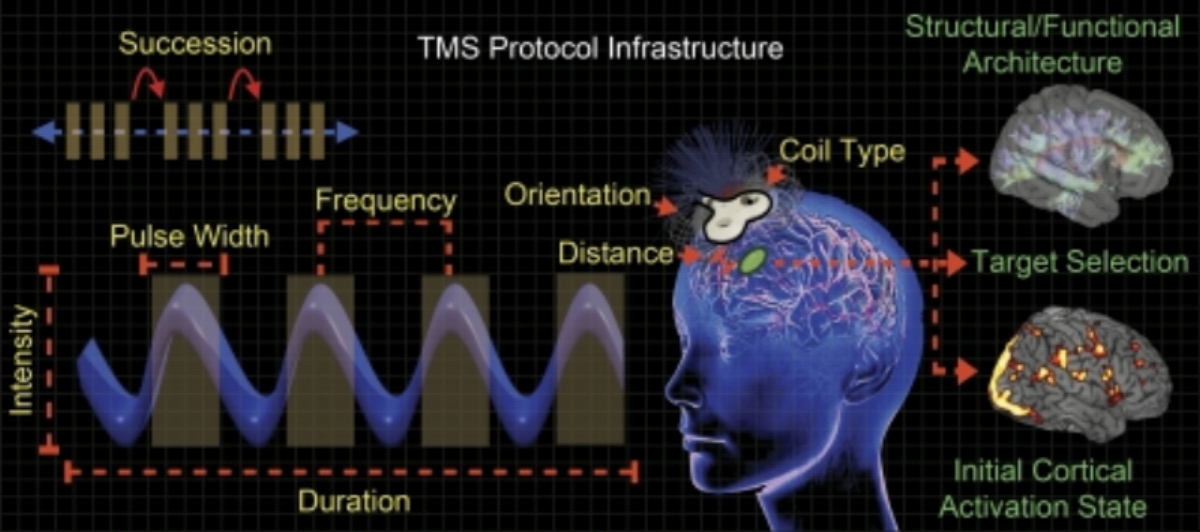
You may be just learning about it,
but TMS is NOT "new" or "experimental"
Time Line of Events in the history of TMS
1985 Barker & Jalinous Letter to Lancet introducing technology
1994 Mark George, MD Published first observations about using TMS for Treatment of depression
2007 4,392 Studies have been published (MedLine). In addition to Depression, TMS is found to be effective for other psychiatric and neurological conditions. Of the 60,000 people who have had TMS, only 2 experienced seizures. Improved safety measures to prevent seizures are introduced. (No seizures have been reported since 2008) Other side effects such as headache or skin discomfort is mild and usually lasts less than 15 minutes. Long term follow up 2, 3, 4 and 10 years shows safety (no late onset problems) and long lasting positive effects. TMS is demonstrated to be 50-100 times safer than treatment with medication.
2008 FDA approves TMS as treatment for Major Depression
2010 the journal: Brain Stimulation - founded by Sackheim & others, publishes 1st issue,
2013 FDA Approves second device (BrainsWay) as treatment for Major Depression
2015 FDA Approves third (MagSTim) and 4th device (MagPro)
By 2015 more than 11,000 scientific studies and reviews have been published. The fact that TMS is safe and effective is a settled issue.