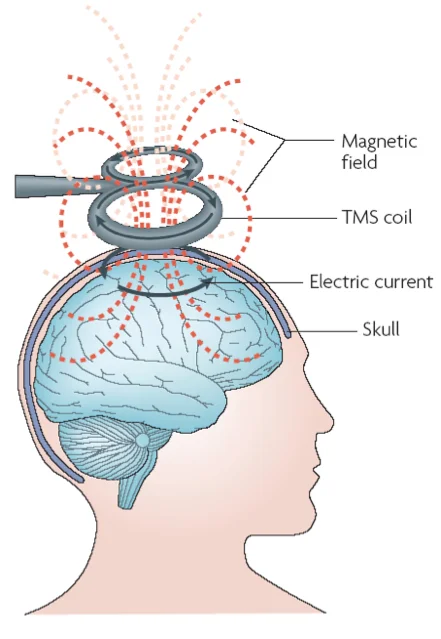
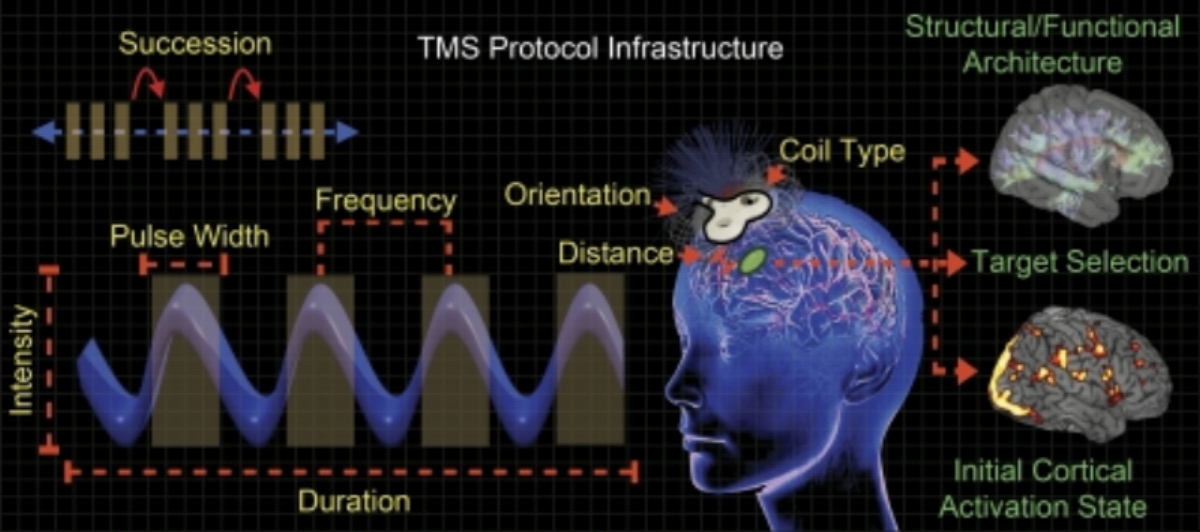
Is a remarkable new treatment for depression and other psychiatric and neurological disorders. It has been used since the early 1990s, and was FDA approved in 2008. Long-term follow-up of patients who TMS has shown no long-term negative effects. The standard course of treatment involves six weeks of daily sessions five days per week. Each treatment lasts 8 to 40 minutes.
Eligible patients include anyone who does not have a history of seizures and does not have any iron-like metal in their brain.
In addition to improving symptoms, other beneficial effects have been observed. These include improved memory, executive function and reduced impulsivity. In some cases patients who have received magnetic therapy for depression have noticed that their desire to drink alcohol, use drugs, or engage in self-destructive behavior has been reduced or disappeared.
Using a longer course of treatment (three or four months) some clinicians have reported significant reduction in symptoms of autism with some children becoming symptom free. We have seen remarkable reduction of symptoms and improvement of function in Parkinson's Disease also.
As with other forms of brain stimulation, the benefits of treatment are usually permanent meaning ongoing treatment can be reduced (Fewer medications 58%) or eliminated (no medications 37%).
About 2.5% of patients who do a six week course of treatment require an additional set of weekly treatments for one month and monthly treatments for four months.
Approximately one in 20 patients who undergo the treatment notice no benefit. Fortunately these individuals can now be identified before beginning treatment by performing a brain scan EEG (Arns 2012).
The cost of treatment ranges from $8,000 to $16,000. Some insurance programs reimburse 40-90%. Currently, Medicare covers this but only in the Eastern half of the US.
In addition to depression treatment, other conditions respond to rTMS including...
Some Conditions That Have Evidence to Support the use of rTMS as Treatment
ADHD (Attention Deficit) (Zaman 2014, Bloch 2010)
Autism Spectrum Disorders (Casanova 2014, Oberman 2013, Oberman 2014, Sokhadze 2014)
Bipolar Disorders (Canali 2014, Zendjidjian 2014, Harel 2010, Dell'Osso 2009, Li 2004, Michael 2004, Nahas 2003)
Generalized Anxiety, Panic Disorder, Social Anxiety & PTSD (White 2015, Paes 2011, Pallanti 2009)
Dementia (Alzheimer's Disease or Vascular) (Cantone 2014, Isaac 2013, Pennisi 2011)
Tinnitus (Ringing in the Ears) (Yilmaz 2014, Forogh 2014, Peng 2012, De Ritter 2013)
Headache (Migraine, Stress & Cluster) (Lipton 2010, Dodick 2010, Brighina 2013, Schwedt 2014)
Parkinson's Disease (Vonloh 2013, Rothwell 2013)
ALS - Amyotrophic Lateral Sclerosis (Di Lazarro 2006, Di Lazarro 2009, Zanet 2008)
Working Memory & Executive Function
Chronic Traumatic Encephalopathy (CTE) in Football Players and other athletes with concussions (Pape 2006, Demirtas-Tatlidede 2012, Rodger 2015, Lu 2015, Li 2015)*
*only Depression is an FDA approved indication, the rest are off-label uses of rTMS
Some clinicians have also reported success with Addiction, Schizophrenia and OCD (Obsessive Compulsive Disorder)
Side effects are mild, 10% experience mild headache (3/10) lasting 5-10 minutes, 1-3% rate of Hypomania or Mania, 1/30,000 chance of seizure (none since 2008)
- • . -
rTMS (Repetitive Transcranial Magnetic Stimulation) is a treatment for patients Who have not responded to pharmaceutical solutions - it is estimated that up to 40% of patients do not benefit from, or cannot tolerate, antidepressant medications - even after repeated attempts.
A recent study has shown that rTMS has a 58% response rate and 37% remission rate and has shown efficacy as a well-tolerated therapeutic option (Carpenter et al 2012)
TRANSCRANIAL MAGNETIC STIMULATION (TMS) FOR MAJOR DEPRESSION: A MULTISITE, NATURALISTIC, OBSERVATIONAL STUDY OF ACUTE TREATMENT OUTCOMES IN CLINICAL PRACTICE
Carpenter et al 2012
Background: Few studies have examined the effectiveness of transcranial magnetic stimulation (TMS) in real-world clinical practice settings. Methods: Forty-two US-based clinical TMS practice sites treated 307 outpatients with Major Depressive Disorder (MDD), and persistent symptoms despite antidepressant pharmacotherapy. Treatment was based on the labeled procedures of the approved TMS device. Assessments were performed at baseline, week 2, at the point of maximal acute benefit, and at week 6 when the acute course extended beyond 6 weeks. The primary outcome was change in the Clinician Global Impressions-Severity of Illness from baseline to end of acute phase. Secondary outcomes were change in continuous and categorical outcomes on self-report depression scales (9-Item Patient Health Questionnaire [PHQ-9], and Inventory of Depressive Symptoms-Self Report [IDS-SR]).
Results: Patients had a mean ± SD age of 48.6 ± 14.2 years and 66.8% were female. Patients received an average of 2.5 (± 2.4) antidepressant treatments of adequate dose and duration without satisfactory improvement in this episode. There was a significant change in CGI-S from baseline to end of treatment (−1.9 ± 1.4, P <.0001). Clinician-assessed response rate (CGI-S) was 58.0% and remission rate was 37.1%. Patient-reported response rate ranged from 56.4 to 41.5% and remission rate ranged from 28.7 to 26.5%, (PHQ-9 and IDS-SR, respectively).
Conclusion: Outcomes demonstrated response and adherence rates similar to research populations. These data indicate that TMS is an effective treatment for those unable to benefit from initial antidepressant medication. Depression and Anxiety 29:587–596, 2012